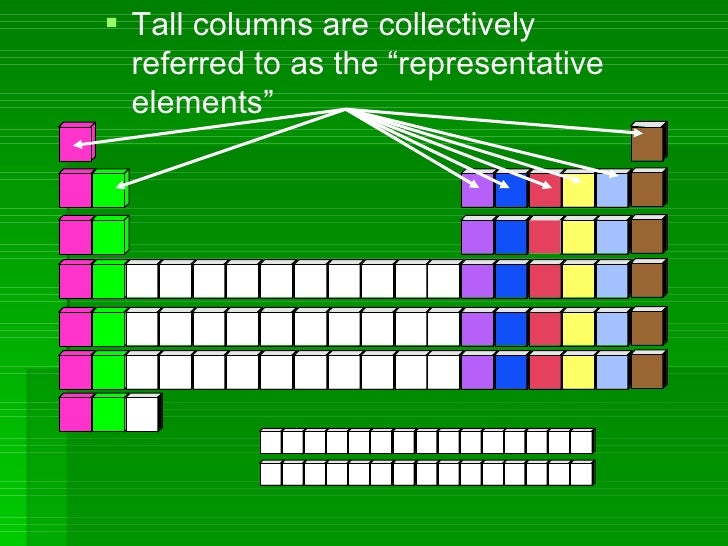
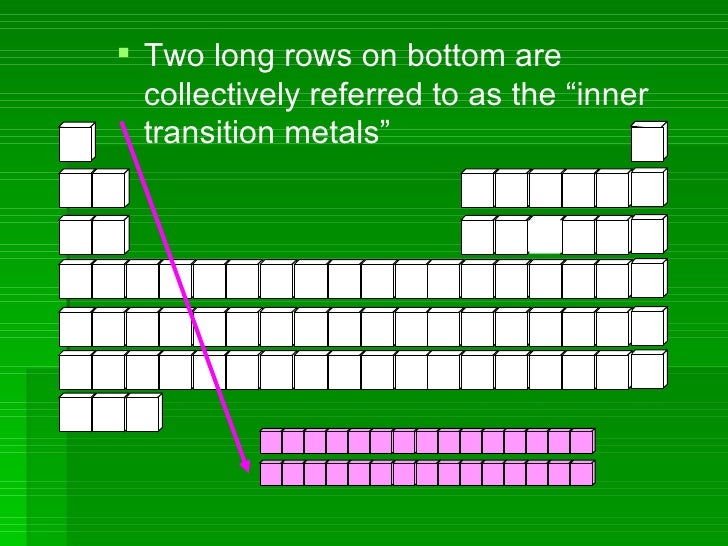
Periods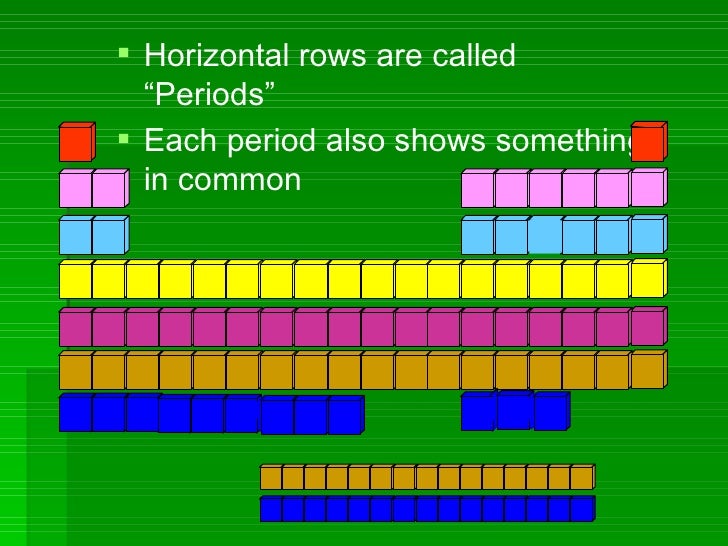

Rows of elements are called periods.
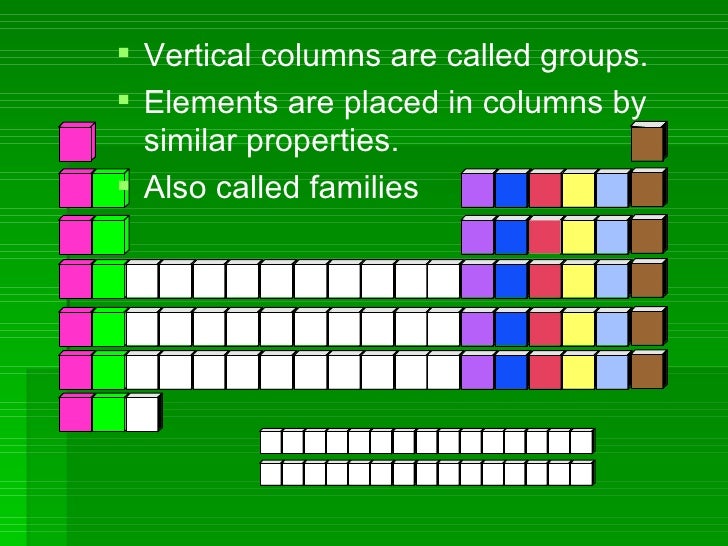
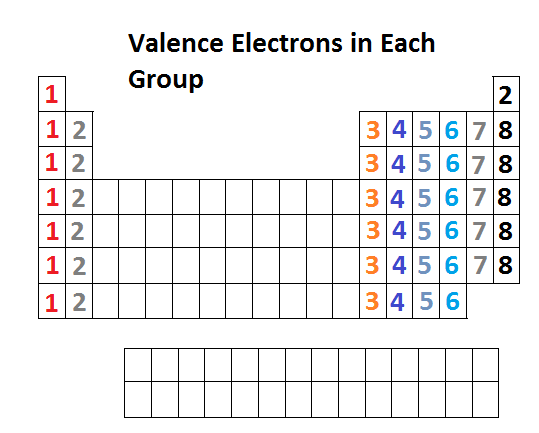
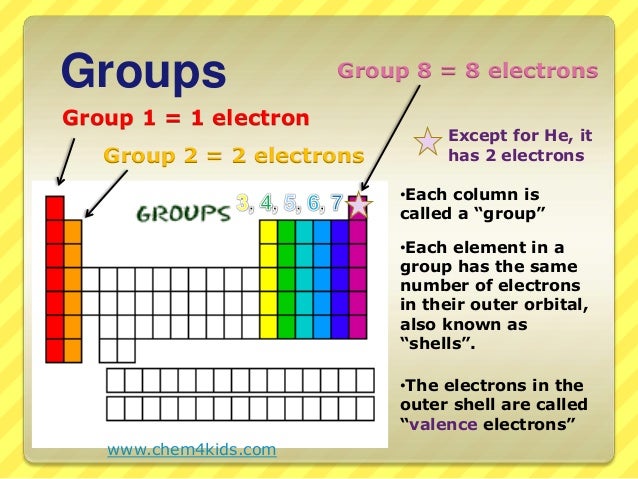
Groups

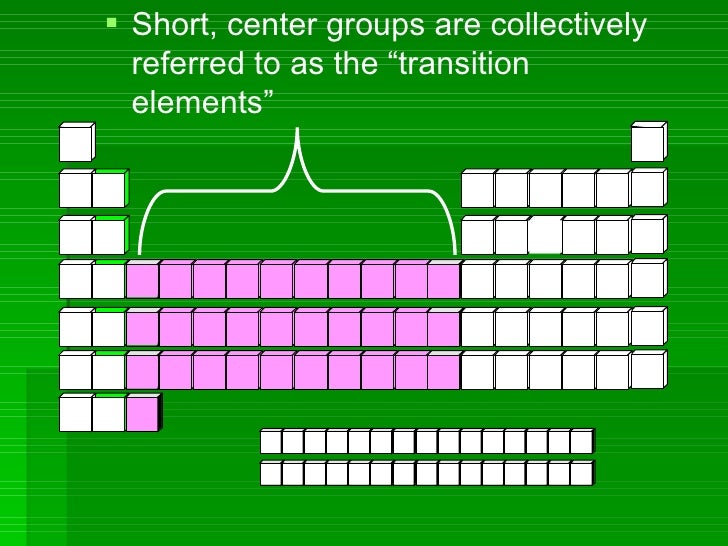
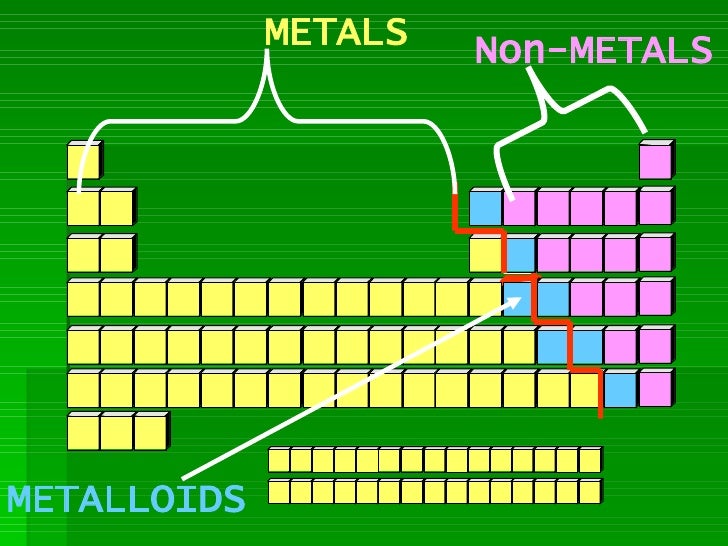
CHARGES ON IONS AND THE PERIODIC TABLE.
The ion formed by an element can be worked out form the element's position in the periodic table . The elements of group IV and and group VIII generally do not form ions.

QUESTIONS
1.In the given periodic table mark
a. one element in group 4
b.a noble gas
c.a transition metal

2.How does the metallic and non-metallic nature of the elements change across period 3 of the periodic table?



























Displacement reactions
Uses of Halogens:
.
plastics Teflon.
and refrigerants.
http://chemed.chem.purdue.edu/genchem/topicreview/bp/ch12/trans.php
http://www.bbc.co.uk/schools/gcsebitesize/science/add_edexcel/periodic_table/groupsrev7.shtml
http://chemwiki.ucdavis.edu/Inorganic_Chemistry/Descriptive_Chemistry/Elements_Organized_by_Block/2_p-Block_Elements/Group_18%3A_The_Noble_Gases/1Group_18%3A_Properties_of_Nobel_Gases
http://chemistry.about.com/od/elementgroups/a/noblegases.htm